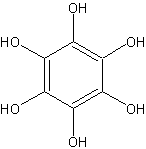

Stannous chloride dihydrate (100 g, 0.44 mole) is added in one portion to a boiling, mechanically stirred solution of 10 g (0.058 mole) of tetrahydroxy-p-benzoquinone in 200 ml of 2.5 N hydrochloric acid. In a few minutes, the red color of tetrahydroxy-p-benzoquinone disappears and long, needle-like crystals of hexahydroxybenzene begin to form. Concentrated hydrochloric acid (250 ml; specific gravity 1.08) is added, and the stirred suspension is heated to boiling, mixed with 600 ml of concentrated hydrochloric acid, cooled in ice, and filtered with suction on a fritted-glass filter. As much solvent as possible is removed by suction, without exposure to air, by covering the filter with an inverted funnel and introducing nitrogen, and by using a polyethylene dam to remove the final traces of the solvent.
For purification, the crude product is dissolved in 450 ml of hot 2.5 N hydrochloric acid containing 3 g of stannous chloride dihydrate. Decolorizing carbon is added, the hot suspension is filtered, the insoluble matter is washed with 75 ml of hot water, and the combined filtrate and washings are mixed with 1 liter of concentrated hydrochloric acid and cooled in ice. The resulting crystals are collected on a fritted-glass filter, in an atmosphere of nitrogen, washed with 100 ml of a cold, 1:1 mixture of ethanol and concentrated hydrochloric acid, and dried over sodium hydroxide in a vacuum desiccator to give 7 to 8 g (about 75%) of colorless crystals which do not melt when placed on a hot plate at 310 °C.
Hexahydroxybenzene can also be recrystallized from 2-methoxyethanol by the addition of benzene; the crystals are separated, washed with acetone, and dried in a vacuum desiccator. The yield is about the same as in the previous method.
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru