Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
1,3-dinitrobenzene
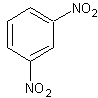
skc-fileSynonyms:
m-dinitrobenzene
Group of substances:
organic
Physical appearance:
colorless rhombic crystalsEmpirical formula (Hill's system for organic substances):
C6H4N2O4Structural formula as text:
C6H4(NO2)2Molar/atomic mass: 168.11
Melting point (°C):
89.57Boiling point (°C):
300Solubility (g/100 g of solvent):
acetic acid: 21.7 (23°C) [Ref.]
acetone: 72.365 (15°C) [Ref.]
benzene: 39.45 (18.2°C) [Ref.]
benzene: 195.89 (50°C) [Ref.]
bromobenzene: 22.7 (20°C) [Ref.]
bromobenzene: 35.9 (30.5°C) [Ref.]
bromobenzene: 121.7 (58°C) [Ref.]
butyric acid: 8.9 (15.5°C) [Ref.]
carbon disulphide: 1.35 (17.6°C) [Ref.]
carbon tetrachloride: 1.18 (16.2°C) [Ref.]
chloroform: 32.4 (17.6°C) [Ref.]
chloroform: 52.4 (32°C) [Ref.]
chloroform: 153.2 (57°C) [Ref.]
diethyl ether: 9.4 (15°C) [Ref.]
ethanol 96%: 3.5 (20.5°C) [Ref.]
ethanol 96%: 11.49 (50°C) [Ref.]
ethyl acetate: 36.27 (18.2°C) [Ref.]
ethyl acetate: 148.44 (50°C) [Ref.]
formic acid: 10.6 (15.5°C) [Ref.]
hydrogen fluoride : very soluble [Ref.]
methanol: 6.75 (20.5°C) [Ref.]
methanol: 11.08 (50°C) [Ref.]
propanol: 2.4 (20.5°C) [Ref.]
propionic acid: 15.54 (23°C) [Ref.]
pyridine: 106.2 (20°C) [Ref.]
sulfuric acid 70%: 0.6 (0°C) [Ref.]
sulfuric acid 70%: 0.76 (25°C) [Ref.]
sulfuric acid 70%: 1.01 (50°C) [Ref.]
sulfuric acid 70%: 3.09 (100°C) [Ref.]
sulfuric acid 90%: 7.7 (0°C) [Ref.]
sulfuric acid 90%: 8.6 (25°C) [Ref.]
sulfuric acid 90%: 11.2 (50°C) [Ref.]
sulfuric acid 90%: 28.7 (100°C) [Ref.]
toluene: 30.66 (16.2°C) [Ref.]
water: 0.0068 (13°C) [Ref.]
water: 0.0496 (50°C) [Ref.]
water: 0.317 (99°C) [Ref.]
Numerical data:
Heat of Explosion (kJ/kg): 3430
Detonation velocity (m/s): 6100
Synthesis 1:
Reference: Гитис С.С., Глаз А.И., Иванов А.В. Практикум по органической химии: Органический синтез. - М.: Высшая школа, 1991 pp. 11017 ml of concentrated sulfuric acid is poured into the round-bottom flask, then gradually, with stirring 12,5 ml of concentrated nitric acid is added. 4,1 ml of nitrobenzene is added to this mixture dropwise, then the flask is closed by a cork with air condenser. The contents of the flask with frequent stirring is heated for one hour on a water bath. After this a test on a completion of the reaction is made, for this purpose several drops of the reaction mixture is placed into a test tube with cold water. The reaction mixture should solidify into crystalls of m-dinitrobenzene without any impurities of oily nitrobenzene. If nitrobenzene still presents, the mixture is heated once again for 10-15 minutes.
Cooled reaction mixture is poured with stirring into a beaker with cold water, solidified m-dinitrobenzene is filtered out and washed with water. The wet product is placed into a beaker with 50 ml of hot water and melted. This operation should be done twice. After cooling m-dinitrobenzene is filtered out and pressed in filter paper. m-dinitrobenzene is purified by a recrystallisation from ethanol (1 gramm of the product requires 7 ml of alcohol).
The yield is 5-6 gramms (75-90% from theory), melting point is 89,5 degrees Celsius.
Dissociation:
pKa (1) = 16.8 (25°C, water)
Standard molar enthalpy (heat) of formation ΔfH0 (298.15 K, kJ/mol):
-27 (s)Molar heat capacity at constant pressure Cp (298.15 K, J/(mol·K)):
197.5 (s)References:
- Seidell A. Solubilities of organic compounds. - 3ed., vol.2. - New York: D. Van Nostrand Company, 1941. - pp. 347-349
- Urbanski T. Chemistry and technology of explosives. - vol.1. - Warszawa, 1964. - pp. 233-248
- Yalkowsky S.H., Yan H. Handbook of aqueous solubility data. - CRC Press, 2003. - pp. 211
- Гитис С.С., Глаз А.И., Иванов А.В. Практикум по органической химии: Органический синтез. - М.: Высшая школа, 1991. - pp. 110 [Russian]
- Справочник по растворимости. - Т.1, Кн.2. - М.-Л.: ИАН СССР, 1962. - pp. 1323, 1364, 1390-1391 [Russian]
- Справочник химика. - Т. 2. - Л.-М.: Химия, 1964. - pp. 508-509 [Russian]
- Хмельницкий Л.И. Справочник по взрывчатым веществам. - Ч.2. - М., 1962. - pp. 404-412 [Russian]
What information do you need?
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru

